
Biostatistics
Our Biostatistics services form the backbone of successful clinical trials and drug development programs, transforming complex data into meaningful insights. As a full-service Contract Research Organization (CRO), we provide expert statistical support to ensure the scientific integrity, regulatory compliance, and success of your clinical research.
+ Read More
Laboratory Services
Our Laboratory Services are designed to deliver high-quality, timely, and accurate data that supports your clinical trials and drug development programs. As a full-service Contract Research Organization (CRO), we offer cutting-edge laboratory solutions tailored to meet your specific needs across a range of therapeutic areas.
+ Read More
Clinical Safety & Pharmacovigilance
At GGAC Research, safety comes first. As a full-service Contract Research Organization (CRO), we provide comprehensive Clinical Safety & Pharmacovigilance solutions to ensure the well-being of participants and compliance with global safety standards throughout the lifecycle of your product.
+ Read More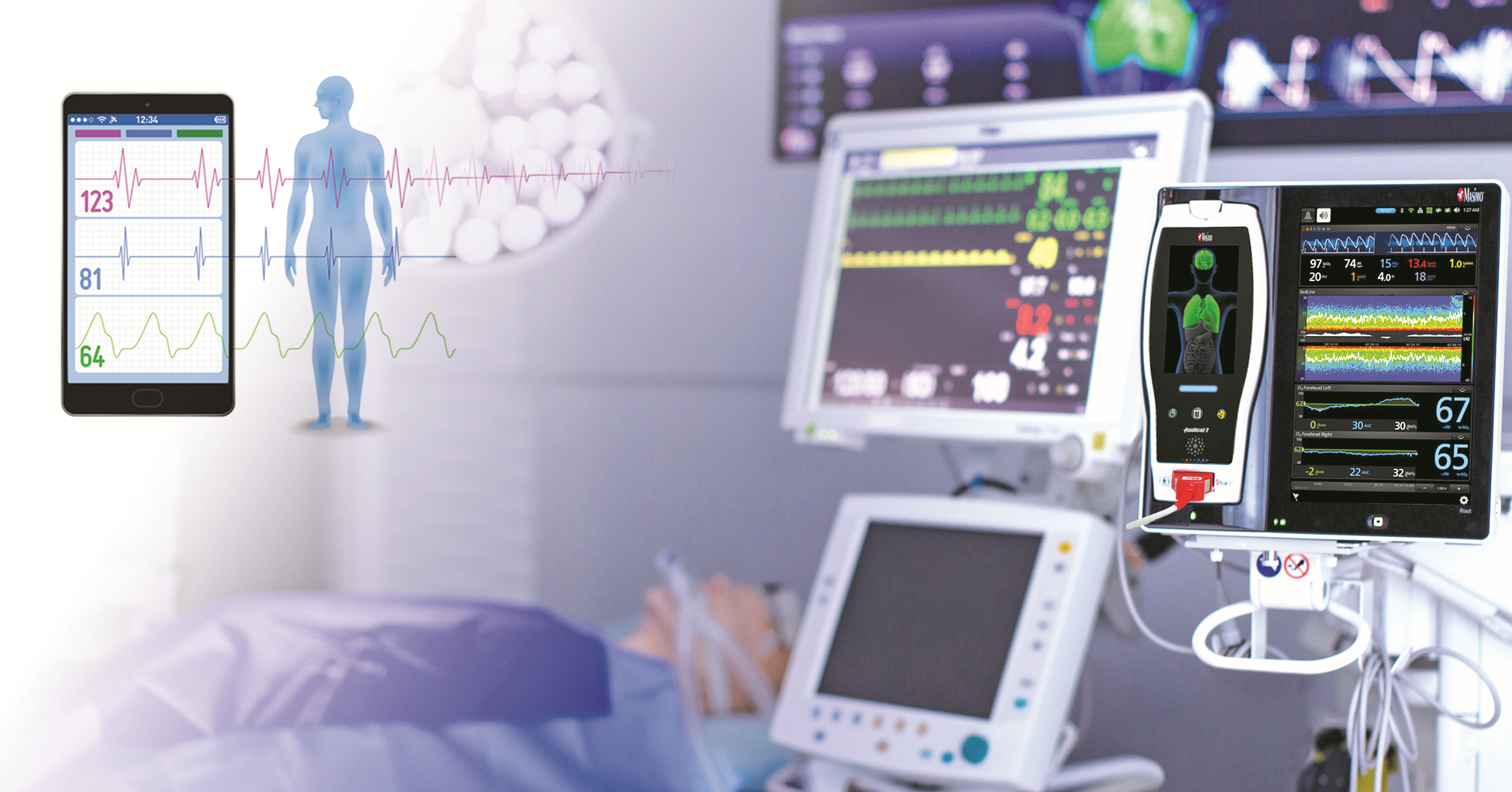
Medical Monitoring
we recognize that clinical trials demand vigilant oversight to ensure participant safety, data accuracy, and protocol adherence. Our Medical Monitoring solutions are a cornerstone of our full-service Contract Research Organization (CRO) offerings, providing real-time expert guidance and oversight to support the success of your clinical trials.
+ Read More
Drug Development
At GGAC Research, we turn your innovative ideas into transformative therapies. As a trusted full-service Contract Research Organization (CRO), we offer comprehensive Drug Development solutions that take your product from concept to market with precision, efficiency, and compliance.
+ Read More
Clinical Trial Management
As a full-service Contract Research Organization (CRO), our Clinical Trial Management solutions are designed to streamline every phase of your clinical research journey, ensuring efficiency, compliance, and impactful outcomes.
+ Read More